Have you ever purchased a skin care product with great optimism that it will solve all of your skin appearance concerns only to find that after using it for months your skin had not improved at all? And instead of looking better your skin looked worse because the product was actually causing irritation. This is a common problem that is typically due to skin care companies being more interested in developing a product with ingredients that make a good marketing story rather than building a formulation based on scientific data.
THREE BASIC “RULES” FOR DEVELOPING EFFECTIVE TOPICAL PRODUCTS
1
Use ingredients that have been scientifically PROVEN to provide benefits to the skin.
2
Use ENOUGH of the ingredient in the formulation to be effective when used topically.
3
Design the formulation to optimize stability of the beneficial ingredients and to ensure that these ingredients are delivered efficiently to the skin.
Step 1: Ingredient Characterization
Pharmaceutical companies that specialize in developing topical dermatology drug products evaluate potential “active” compounds using a rigorous cell and molecular biology based drug screening program. This rigorous multi-step screening program determines:
- The types of skin cells affected by the potential drug compound.
- The specific cellular and molecular signaling pathways and cellular events in each cell type that are regulated by the compound.
- The effect of each bioactive compound on the expression of skin specific genes (over 5000 genes can be screened at one time) to determine any NEGATIVE as well as POSITIVE effects on skin cells.
- The effect of each compound on the production and secretion of inflammatory hormones and on proteins involved in skin structure.
- The potency of each compound in regulating cellular and molecular processes.
Different screening strategies are utilized by different laboratories to determine the effects of a compound on either: 1) inflammation pathways, or 2) anti-aging events. For example, a compound being studied for anti-cancer activities, might be tested on cancer cells in culture for its ability to halt cell proliferation or, alternatively, to trigger the cancer cell to undergo apoptosis (cell death). For dermatology applications, screening is typically carried out with human keratinocytes, fibroblasts and monocytes. Potential “bioactive” compounds are often screened for their ability to block the production of and/or action of PGE-2 (Prostaglandin E-2) since this inflammatory compound is involved in sunburn and many other skin problems, and most importantly, is strongly linked to the progression of skin cancer, including basal, squamous cancer and melanoma.
Step 2: Determination of Optimum Concentration to Use in a Topical Formulation
Once a potential “bioactive” compound is identified, the next step in product development is to determine the potency of the compound. Is it effective in blocking the inflammatory event or aging event at a concentration that could realistically be used in a topical formulation? For example, screening with skin cells in culture might have identified a compound that blocks PGE-2 when used at a concentration of 0.5% in cell culture medium. But a good rule of thumb to use in topical formulation development is that one needs to multiply the concentration found to be effective in cell culture screening studies by at least 100-200 when formulating the “bioactive” into a topical product. So in our simple example, a compound that is only effective at 0.5% in cell culture would have to be used at 50% in a topical formulation, which is, of course, not feasible. Typically, topical formulations contain concentration of active ingredients that range from 0.05% to 5%. That means that for cell culture screening studies, the “active” should be effective in blocking inflammation or demonstrating an anti-aging effect at a concentration of somewhere between 0.00025% and 0.025%.
Do all skin care companies adhere to this approach for developing formulations with the correct and optimal amount of “active” ingredient? The answer, most of the time, is no. In fact, many skin care companies incorporate a scientifically proven “active” ingredient into a formulation at a concentration that is so LOW the ingredient can’t possibly provide any skin benefits. When this is done, formulation chemists refer to this product development approach as “fairy dusting”. The company adds tiny amounts of an excellent ingredient to the product for the sole purpose of marketing claims and NOT to improve the skin.
Step 3: Design the Formulation to Optimize Stability of “Active” and Its Delivery to the Skin
Although results from screening studies may show that a “drug candidate” is very effective at low concentrations, there is no guarantee that the compound will be beneficial when applied topically. Such potential problems as compatibility with formulation ingredients, inability to penetrate through the stratum corneum, instability due to temperature, pH, oxidation, etc. can prevent a potential “active” drug candidate from ever being developed into a product. One of the first tests pharmaceutical companies conduct on any potential drug candidate is to incorporate the “active” into a topical formulation and determine its stability under a variety of “stress” conditions. Once a formulation is developed that maximizes the stability of the active ingredient, the formulation is then tested for its ability to deliver the “active” efficiently to the skin and across the stratum corneum. For any potential topical drug formulation to be effective in treating a skin problem, the “active” must be able to pass into the stratum corneum, and not remain on the surface. The delivery of any ingredient across the skin's surface can be verified by laboratory tests that use actual human skin. This skin penetration test is called Skin Percutaneous Absorption Analysis, and the device most often used to carry out this analysis is a Franz Cell.
A Franz cell is a specially designed apparatus that allows for the determination of the precise amount of any compound that can penetrate through either human skin (the preferred method), or through a synthetic membrane. To obtain the most useful and predictive data, human skin is used. A diagram of a Franz cell unit is shown here.
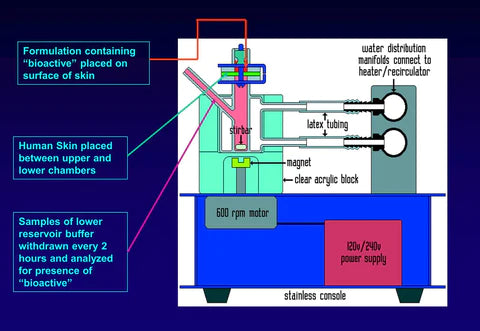
As shown in the diagram, a section of human skin is sandwiched between an upper donor chamber and a lower receptor chamber. The lower receptor chamber is filled with fluid and is equilibrated to a temperature of 37 C (normal body temperature) by the circulating water jacket. The test formulation is applied to surface of the skin through the upper chamber and samples of fluid in the lower receptor chamber are taken at specific time intervals (ex. 4, 8, 12 and 24 hours). The samples from the receptor chamber are then analyzed by HPLC to quantify the amount of any ingredient (bioactive) that has penetrated through the skin and entered the receptor chamber.
A topical formulation containing a potential drug candidate compound that successfully completes these 3 development steps will likely be effective in addressing whatever skin problem it has been designed to treat. However, the only way to know for sure that a product will be effective is to run clinical studies using an independent testing site to evaluate the product's effectiveness. There are typically two types of clinical studies run on dermatology drug products. The simplest study design involves giving the product to patients to use and then have the clinical investigator assess the effect of the product in improving a particular skin condition after one to two months. Since the patients and clinical investigator know that the topical formulation contains the “active” ingredient and know what the “active” is, the study is referred to as an “open label study”. The goal of an open label study is simply to determine that the skin condition of the patients using the product improves after a month of use. It is not designed to prove conclusively that it is the “active” ingredient, and not the other ingredients in the formulation, that is causing the improvement of the skin. To show that the effective treatment of any skin disorder is due specifically to the drug “active” in the formulation, “blinded” placebo controlled clinical studies must be carried out. In this type of study two patient groups are enrolled. One group is given the test formulation that contains the proprietary ingredient while the other group receives exactly the same formulation that does not contain the proprietary ingredient. In this study neither the clinical investigator nor the patient knows which formulation is the “placebo” and which has the “active” drug in it. Although both “open label” and placebo controlled studies can provide useful information on the effectiveness of a given product in improving a skin condition, if the objective of the clinical study is to evaluate the efficacy of a specific proprietary ingredient (e.g. a potential drug), then placebo controlled studies need to be used.
Although DermaMedics products are developed for the cosmetic or OTC drug market, the same “rules” that pharmaceutical companies use to develop products are followed during product development. This development strategy ensures that each product will perform optimally to rapidly improve skin appearance and do so without causing irritation to even the most sensitive skin.