ANTIOXIDANTS INACTIVATE FREE RADICALS THAT DAMAGE AND AGE YOUR SKIN
FREE RADICALS DAMAGE PROTEINS, LIPIDS, CELL MEMBRANES AND DNA AND ALSO TRIGGER AN INFLAMMATORY RESPONSE. THIS INFLAMMATION WILL DAMAGE AND AGE YOUR SKIN. REGARDLESS OF WHAT “MIRACLE” ANTI-AGING INGREDIENT IS IN A SKIN CARE PRODUCT, IF IT DOESN’T CONTAIN POTENT ANTIOXIDANTS TO INACTIVATE FREE RADICALS YOUR SKIN WILL LOSE ITS HEALTHY AND YOUTHFUL APPEARANCE!
Everyone has heard of antioxidants and everyone knows that antioxidant rich foods are good for you. However, not too many people actually know what an antioxidant is, how it works, and why it is “good” for you. Understanding what antioxidants are and how they work is not difficult. They are simply molecules (like vitamin C) that can prevent the oxidation (a loss of electrons) of other molecules. When molecules, like proteins, DNA or lipids lose an electron (undergo oxidation), they are structurally damaged (mutated) and no longer function normally, These mutations can lead to a wide number of medical problems including cancer, autoimmune disorders, aging, inflammatory skin diseases, rheumatoid arthritis, and cardiovascular disease, to mention just a few. So just what causes molecules to undergo “oxidation’ and lose electrons? The answer is that FREE RADICALS carry out these oxidation events. But what are free radicals and how do they act to cause damage to proteins, DNA, lipids and other important molecules in your body?
FREE RADICALS AND REACTIVE OXYGEN SPECIES (ROS)
Free radicals are molecules or atoms with unpaired electrons. They are produced constantly in the body. As you’ll recall from high school chemistry, the nucleus of every atom is surrounded by electrons that travel around the nucleus in layers, called orbitals or “shells”. Each shell can hold a given number of electrons and when this number is reached additional electrons begin filling new “shells”. For example Oxygen (O) has 8 electrons and, of course, 8 protons in the nucleus to give an atomic mass of 16. The first electron shell can hold a maximum of 2 electrons while the second shell can hold 8 electrons. But since there are only 6 electrons left after 2 of the oxygen electrons filled the first shell, the second shell has 2 open spaces for 2 more electrons. Because of the open spaces, oxygen is actually considered a “biradical”. However, unlike other free radicals, oxygen is fairly stable (although not completely stable) with just 6 electrons in its outer shell because each electron has another one to “pair” with. If an atom has an electron with no partner to “pair” with, the atom becomes much more chemically reactive than those with paired or completely filled electron shells, and this “reactive” atom is called a “free radical”. Because atoms always want to reach a state of maximum stability, they will try to fill any “missing” electron in their “unfilled” shell by “stealing” an electron from another molecule. When the “target” molecule loses an electron to the free radical it then, in turn, becomes a free radical and must find a “donor” it can steal an electron from. Thus, an oxidative chain reaction begins that results in extensive damage to cellular proteins, lipids, membranes and DNA. These damaged molecules don’t function normally and this abnormal functioning can cause serious damage to all cells and organs in the body, and even lead to cancer.
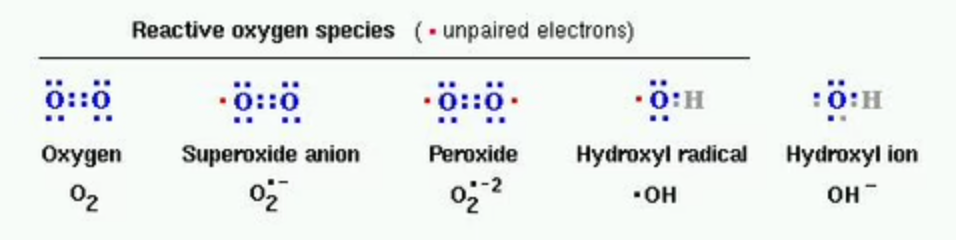
Most free radicals in biological systems are derivatives of oxygen. The most common oxygen radicals in the body are the superoxide anion (O•2−) and the hydroxyl radical (•OH−). These free radicals are commonly called Reactive Oxygen Species (ROS), and when the skin is stressed by environmental stressors such as UV radiation or pollution, this “oxidative stress” leads to the formulation of high levels of ROS. Below is an image that shows several ROS.
To give you one very important example of how dangerous mutations induced by free radicals can be, consider the protein p53. This protein is known as a “tumor suppressor” because of its role in monitoring the health of the cells in your body. If, for example, your skin receives too much UV radiation (sun) exposure, the free radicals that are produced by the UV radiation can cause DNA mutations. Some of these mutations may cause skin cells to become cancerous (basal cell cancer, squamous cell cancer and melanoma). P53 continuously monitors cells for DNA mutations and if it finds one, p53 can stop the cell from dividing. It will then try to repair the DNA damage, or if the repair fails, p53 will trigger cell death, thus ridding the body of a potential cancer cell. But what happens if the UVR induced free radicals not only cause mutations in many parts of the DNA but also damage the region of the DNA that carries the gene for p53? If this occurs the p53 protein that is produced from this mutated gene is abnormal and loses its “surveillance and repair” ability? When this happens, lethal DNA mutations are not repaired and the mutated cell is not destroyed. It can survive and become a cancer cell. In fact, mutations in p53 have been found in EVERY type of cancer and in some cancers, like melanoma, 90% of these tumours have changes in the p53 protein. This one example should convince you that by damaging DNA and proteins, free radicals can cause serious health problems.
FREE RADICALS INCREASE NATURALLY WITH AGE
Our bodies are stressed all day long by environmental factors like UV rays from the sun, pollutants, and pesticides. This stress results in increased free radical production. In addition, as we age, the cells of our body unfortunately naturally generate higher levels of free radicals than they did when we were young. Not only do these free radicals irreversible damage the body’s proteins, lipids, cell membranes and DNA, they also trigger an inflammatory response in the body, often referred to as “smoldering inflammation”. This increased inflammation as we get older is responsible for various age-related problems such as arthritis, Alzheimer’s disease, cardiovascular disease, diabetes, cataracts, cancer, and of course, skin aging. In the case of skin, the production of free radicals plays a significant role in skin aging and even skin cancer. Free radicals produced in the skin in response to sun exposure, or to chronological aging trigger the production of high levels of inflammatory mediators that cause extensive damage to the structural components of the dermal matrix, resulting in a loss in collagen, a loss of barrier function, increased skin wrinkling, a loss of elasticity, an increase in abnormal cells (actinic keratosis and skin cancer) and an increase in irregular pigmentation.
ANTIOXIDANTS PROTECT CELLS FROM FREE RADICAL DAMAGE AND REDUCE SKIN AGING
Everyone knows that antioxidants are “good for you”, but why are they so important to include in your daily diet and in your skin care products? Antioxidants protect cells from free radical/ROS damage by either donating an electron to a free radical thereby stabilizing it and halting the chain reaction of destruction or by accepting the one unpaired electron the free radical is carrying, and by doing so stabilize the free radical and prevent it from interacting with and damaging proteins, DNA and lipids. By donating an electron to the free radical to stop the chain reaction, the antioxidant itself becomes a free radical. However, because of its structure, the antioxidant is far less reactive than other radicals. If the antioxidant is relatively large, the effect of the unpaired electron is “diluted” along its structure making it “non-reactive”.
Antioxidants can prevent free radical damage to the skin in several ways:
1) By inactivating free radicals, antioxidants block the ROS “electron stealing” chain reaction that can cause damage to collagen and elastin, a loss of skin elasticity, and an increase in skin wrinkling. In additions by inactivating free radicals, antioxidants prevent mutations in the DNA of skin cells that can lead to skin cancer.
2) By inactivating free radicals, antioxidants PREVENT them from stimulating inflammatory signaling pathways that lead to the activation of inflammatory genes. For example, by blocking these pathways, antioxidants prevent the activation of the genes for the enzymes, MMPs, that destroy collagen, elastin and other important skin proteins.
3) Antioxidants reduce the formation of AGEs (Advanced Glycation End products) that are produced, in part, by free radicals. Glycation refers to the irreversible linking of sugar (glucose) to proteins, such as collagen. This linkage alters the structure of proteins rendering them incapable of normal function. In addition, AGEs produce more free radicals and also trigger inflammation, leading to skin damage.
Unfortunately, overall aging of the body is free radical related and this has led to the “free radical theory of aging”. When we’re younger the antioxidant system in our bodies can “mop” up free radicals and prevent damage, but as we get older these antioxidant systems don’t work as well, and the level of free radicals rises.
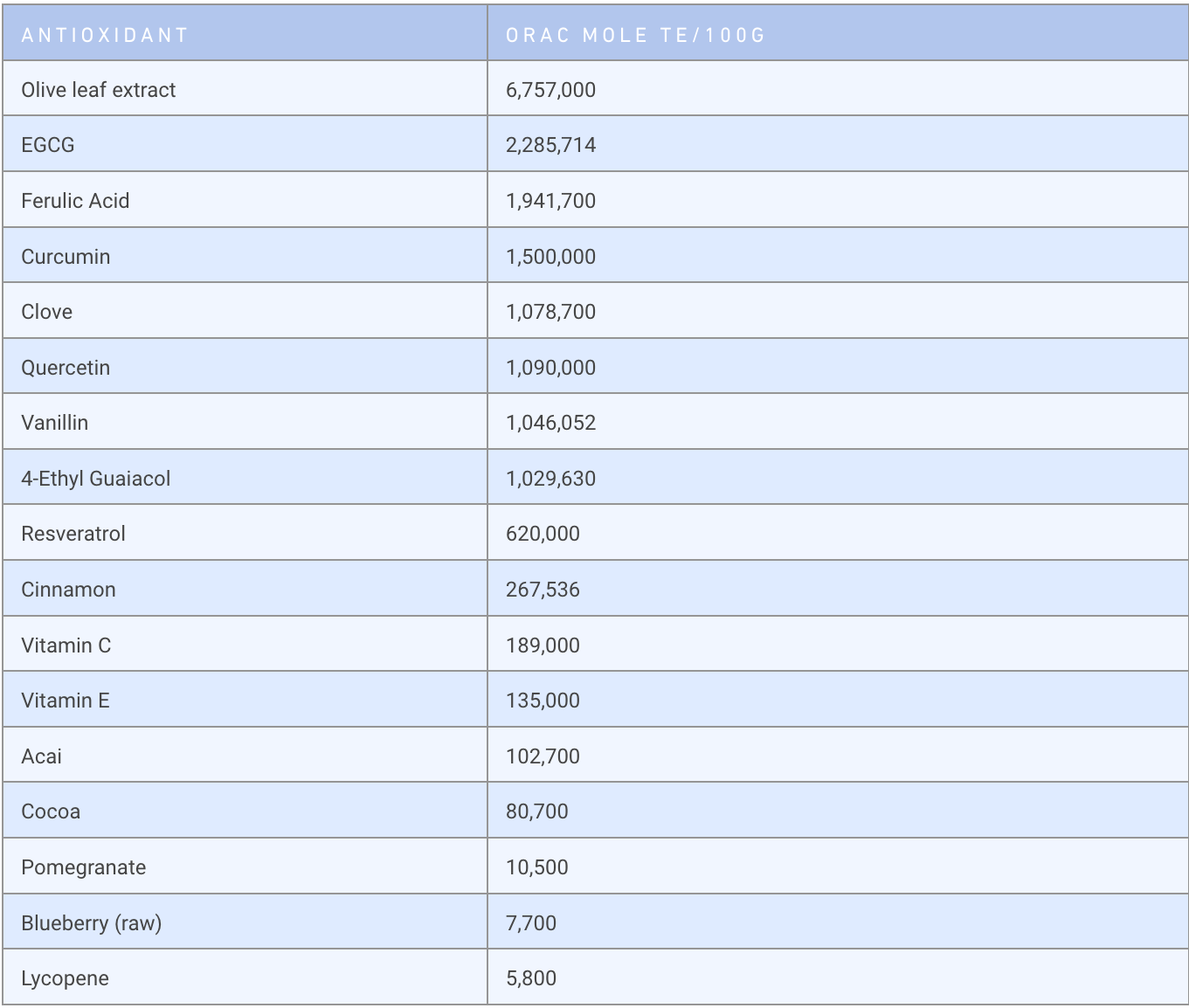
In addition to incorporating antioxidants into one’s diet, another important step to take to protect the skin from the destructive effects of free radicals (ROS) is to use skin care products that contain high levels of antioxidants. Why is this important? The reason is that many antioxidants one consumes with a meal NEVER get out to the skin. If they can’t reach the skin, they can’t block free radicals!. But skin care products that are applied topically can deliver antioxidants across the skin’s surface to fight free radicals. The higher the antioxidant level in a topical product, the more protection that product will provide in blocking free radical damage to the skin.
SOME BOTANICAL ANTIOXIDANTS ALSO HAVE DIRECT ANTI-INFLAMMATORY ACTIVITIES
As discussed above all antioxidants inactivate free radicals and since free radicals trigger inflammation, free radicals INDIRECTLY inhibit inflammation and lower the level of destructive inflammatory mediators. However, since the production of inflammatory mediators in the skin is also stimulated by factors OTHER than free radicals, just having an antioxidant in a product that only blocks free radicals will not completely eliminate inflammation However, there are some very potent antioxidants that not only inactivate free radicals but also DIRECTLY block the activation of inflammatory pathways. Many laboratories both in academia and in industry are now focusing their research efforts toward identifying these powerful botanically derived compounds that display both antioxidant and direct anti-inflammatory properties, and which may prove to be safer than the currently used prescription anti-inflammatories such as corticosteroids and biologics. Many well-known antioxidants have now been shown to have pronounced direct inhibitory effects on cellular signaling pathways that cause inflammation. Some of these antioxidants are well-known and include curcumin (from Tumeric), quercetin (from fruit), EGCG, (from green tea) and resveratrol (from red wine).
Dr. Fuller’s research in his laboratory in the Department of Biochemistry and Molecular Biology at The University of Oklahoma Health Sciences Center focused on investigating the anti-inflammatory activities of many natural antioxidants, like those listed above. And like researchers at other institutions, Dr. Fuller developed a screening program to characterize the biological activities of botanically derived antioxidants, with the goal of finding new and safer ways to address the number one cause of human disease; INFLAMMATION. This screening program is discussed in the section entitled Identifying Natural Ant-inflammatory and Anti-Aging Compounds. Such diseases as MS, arthritis, Parkinson's, Lupus, cardiovascular disease, Alzheimer's, most skin diseases, skin aging, and even cancer all involve are all inflammation based and most, if not all, involve free radicals. Because most pharmaceutical drugs on the market today had their origins in plant derived chemical compounds, research laboratories all over the world are now "going back to nature" to identify potentially useful compounds that are effective in addressing inflammation but which do not have the side effects of synthetic drugs on the market today. Dr. Fuller’s research group conducted years of research to characterize the biological activities of hundreds of botanical antioxidants with the goal of finding those antioxidants that could not only inactivate free radicals, but which could also directly inhibit inflammatory events in the skin. As discussed in the Science Behind DermaMedics section, Dr. Fuller’s research led to the discovery of a family of novel and potent antioxidants that had direct anti-inflammatory activity and these were sold to DermaMedics. The company now owns these patents and makes them available to pharmaceutical partners through licensing agreements.
Antioxidants that can not only inactivate free radicals but can also directly block cellular pathways that cause inflammation are referred to as dual function antioxidants, or Ultra-Antioxidants™. Obviously, an ideal skin care product should contain one or more of these Ultra Antioxidants. DermaMedics®’ patented compounds are antioxidants that have this DUAL inhibitory activity. They block free radical activity and they inhibit the synthesis, secretion and action of many inflammatory mediators produced in the skin. All DermaMedics® products contain one or more of these patented “Ultra Antioxidants”. For more information about DermaMedics®’ patented compounds you can view the patents at: https://www.uspto.gov/) and select Dr. Fuller’s issued U.S. patents (U.S. patent numbers 9,622,950 and 9,616,006).